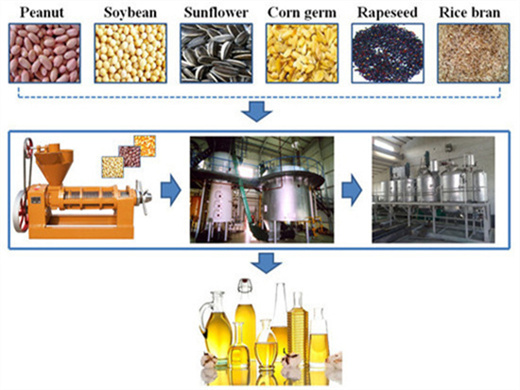
Solvent Extraction Process in Petroleum Oil Refinery
- Usage: Cooking Oil
- Capacity: 22-25 ton/day
- Voltage: 380V/440V
- Dimension (L*W*H): Depends
- Weight: 0 KG
- Guarantee: One year warranty against manufacturing defects.
- Main components: Gearbox
- Oil type: Cooking Oil
- Full Warranty Service: Video Technical Support
- Post Warranty Service: Spare Parts
- Onsite Warranty Service : Field repair and maintenance service
- Raw material: Flax seed
- Product name: Oil pressing machine
- Function: Edible oil manufacturing
- Application: Edible Oil Production
- Keyword: Oil Expeller
- Used for: Edible Oil Manufacturing
The process of transferring a substance from one solvent to another due to differences in solubility or distribution coefficient between the two immiscible (or slightly soluble) solvents is known as the solvent extraction process.
This method is often preferred when the uranium concentration in solution is high (e.g., >800 mg/L [13]); however, there is no practical solvent extraction process developed for carbonate solutions [14]. The basic premise of solvent extraction is to have the leach solution mix with an organic solvent in a large tank.
Overview of Avocado Oil Market in Kenya Freshela Exporters
- Usage: Cooking Oil
- Capacity: 10T-3000T/D
- Voltage: 220V/380V/440V
- Power (W): As per your request
- Dimension (L*W*H): As per your request
- Weight: As per your request
- Certification: ISO9001
- Processing: Batch-type or Semi-continuous
- Electric Consumption: 28Kwh/T Oil
- Softened Water:
- Phosphoric Acid:
- Bleaching Earth Consumption:
- Refining Rate:
- Residual bleaching earth oil content:
- Water circulating Cooling water performance:
- Type of supplier:
- ITEM: machine to produce virgin cooking oil
Avocado Oil Extraction Process in Kenya. Avocado oil like olive oil is extracted from the fruit pulp. This method is known as the cold-pressed extraction method. Kenya produces avocado oil that is high quality and edible. After harvesting, the avocados are taken to the avocado oil processing plant where they are carefully sorted and weighed.
Uses of solvent extraction process ?Solvent extraction is used in the processing of perfumes, vegetable oil, or biodiesel. ?It is also used to recover plutonium from irradiated nuclear fuel, a process which is usually called nuclear reprocessing. ?The recovered plutonium can then be re-used as nuclear fuel.
Chapter 6. Solvent Extraction
- Usage: Edible Oil
- Capacity: 98---100%
- Voltage: 380v
- Dimension (L*W*H): 2100*1500*2600mm
- Weight: 1000 KG
- Main components: motor, pressure vessel, pump, PLC, others, gears, bearings, motor, gearbox
- Oil type: cooking oil
- Certification: ISO9001
- Function: Edible oil manufacturing
- Application: Edible oil production
- Advantage: High performance of oil
- Feature: High oil yield efficiency
- Used for: Commercial oil ejector
- Capacity: 2.8 t/ 24h
- Quality: High Level
- MOQ: 1 Set
- Service after warranty: Online technical support
Solvent extraction is a common technique utilized for both industrial applications and in the laboratory. The technique is successfully applied as a sample preparation procedure for chromatography.
3. Importance of the process: The Solvent Extraction(S.E.) process was first developed as a tool of analytical chemistry. Every metallic element of the periodic table could be virtually separated by this process. Back in 1940’s, S.E. was primarily used to separate nuclear and rare earth elements. However, availability of inexpensive and effective reagents led to the establishment of large
Solvent Extraction: Introduction, Principle, Process ..
- Usage: Edible Oil
- Voltage: 220V/380V/440V
- Power (W): 18.5KW/T
- Dimension (L*W*H): 48m*12M*15M (30TPD)
- Weight: 30 tons
- Material: stainless steel
- Engineers application: 1-2 engineers
- Grade of oil: 1st, 2nd, 3rd
- Environmentally friendly: yes
- Trade methods: Grape seed oil machine
- rate of oil: 20%-98%
There is also a solvent extraction process called the liquid-liquid extraction process. The liquid-liquid extraction process involves the separation of solute from a mixture using two immiscible solvents. Most of the refined edible oils are obtained by this liquid-liquid extraction process. Table of Contents: Introduction of Solvent Extraction
Typical solvent extraction flow sheet. Download Scientific ..
- Usage: Edible Oil
- Capacity: 35~70kg/h
- Power (W): 1.5KW
- Dimension (L*W*H): 900*850*1550 mm
- Weight: 1000kg
- Workers required: 1~2
- Plant: 30~50 square meters
- Business accepted delivery terms: FOB, CFR, CIF, EXW
- Warranty: 1 year
- Packaging: Wooden package
- Suitable objects: rapeseed, peanut, walnut, sesame, etc.
- Manufacturing experience: more than 30 years
Solvent extraction allows for higher throughput and is capable of attaining higher purification but since the amount of solute transfer during solvent extraction is limited by the equilibrium
- What is solvent extraction?
- Solvent extraction is one of the separation methods in which one component or solute present in one phase is extracted into another phase by using a suitable solvent. Thus, the process by which a solute is transformed from one phase to a new phase is known as extraction.
- Which solvent is used in liquid extraction?
- The common extraction solvents used are ether, chloroform, benzene, CCl 4, ethyl acetate, etc. In simple liquid-liquid extraction, the solute is partitioned between two immiscible phases (solvents).In most cases one of the phases is aqueous and the other phase is an organic solvent such as ether, or chloroform.
- What are some examples of solvent extraction techniques?
- Different solvent extraction techniques are applicable across diverse industries. Some examples purification of drugs from natural sources often rely on solvent extraction techniques. Solvent formulations. and substances using extraction methods, such as steam distillation or SFE. colors, and nutraceuticals from raw materials.
- When did solvent extraction become possible?
- After 1870’s , light petroleum fractions have become available and real solvent extraction could be performed. Solid-liquid extraction or leaching is the diffusive transfer of soluble components to a surrounding solvent. The extracted components (oil) can be recovered by simple evaporation of solvent.
- What is a solvent extraction plant?
- The quality of oil is similar to that of expeller oil. Solvent extraction plant consists of a extractor, in which oilseeds and solvents are mixed thoroughly. Then the oil is freed from the solvent in a series of stills and stripping columns with associated condensers. Then oil is cooled and filtered for further storage.
- Which solvent is used for extraction of nonpolar compounds?
- Examples include hexane, ether, and chloroform. These solvents are typically used for the extraction of nonpolar compounds, such as fats, oils, and aryl compounds. solvent for a specific extraction process. be discussed below. compounds based on their relative solubility in two immiscible liquid phases. One phase contains the impurities.